I have been using FiberCell Systems cartridges for the production of recombinant proteins in CHO cells for five years. I routinely produce a half-gram of protein in two months in a volume of less than five liters. This type of production would not be possible using conventional methods. FiberCell Systems hollow fiber bioreactors have made an invaluable contribution to my research.
– Dr. James Arthos, Bethesda, Md.
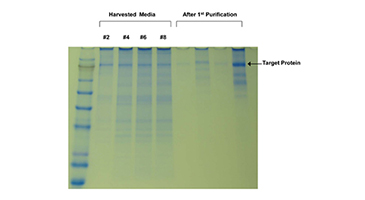
Interleukin 15 receptor complex is one of the ultimate difficult-to-express proteins. It consists of two subunits, held together by hydrophobic interaction and is 45% glycosylated. The IL15-RC heterodimer demonstrates superior pharmacokinetics and in vivo bioactivity compared to the single chain IL15 expressed in e. coli. Expression in standard cell culture systems is problematic. Efficient production of this noncovalently linked but stable heterodimer in HEK293 cells is demonstrated in a 5 kD MWCO HFBR.
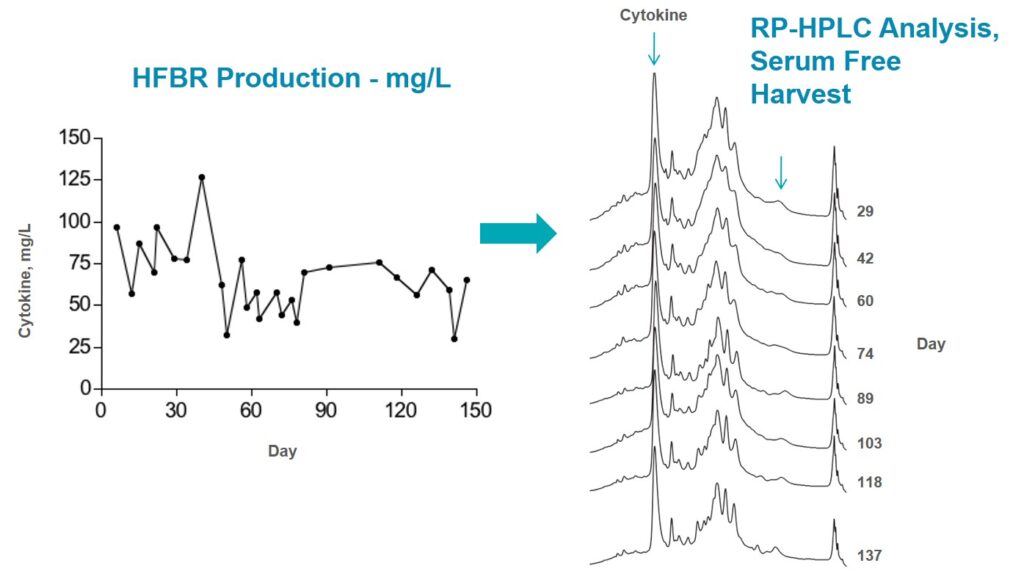
Cell supernatants (20 mL) were harvested daily for up to 5 months and assayed for IL levels by ELISA. This is an important cytokine with potential clinical applications as a lymphocyte growth and activation factor. Although monomeric E. coli-produced IL15 is in the initial stages of clinical testing, this form of the molecule poses multiple challenges for clinical use due to its instability and rapid plasma clearance. HEK293 cells produce correctly folded and glycosylated human IL-RC heterodimeric cytokine that shows greater stability and longer half-life. The superior bioactivity of IL15-RC in the heterodimeric form is the result of the presence of the IL-receptor contributing to increased stability of the protein in vivo. These properties offer the potential to allow lower and less frequent dosing and simpler delivery methods, with increased convenience for both patients and caregivers.
Data above, demonstrating consistent, continuous product of the IL over 150 days of culture, and no change in glycosylation or assembly over this time. Protein research at the laboratory scale is the basis for the development of therapeutic products. It is critical that these be produced in a form that retains all of the characteristics of the final product so that results seen the research lab can be extended to the clinic. Expression of proteins and especially difficult-to-express proteins in mammalian systems is efficient and cost effective in a HFBR. There are many advantages to working with a protein that is correctly folded with tertiary structure intact.