I have been using FiberCell Systems cartridges for the production of recombinant proteins in CHO cells for five years. I routinely produce a half-gram of protein in two months in a volume of less than five liters. This type of production would not be possible using conventional methods. FiberCell Systems hollow fiber bioreactors have made an invaluable contribution to my research.
– Dr. James Arthos, Bethesda, Md.
In 1986 human tissue plasminogen activator (tPA, Activase; Genentech, S. San Francisco, CA, USA) became the first therapeutic protein from recombinant mammalian cells to obtain market approval. Today about 60–70% of all recombinant protein pharmaceuticals are produced in mammalian cells. This is due to the complex nature of required post-translation modifications for these proteins to ensure proper solubility and bioactivity. Like tPA, many of these proteins are expressed in immortalized Chinese hamster ovary (CHO) cells, but other cell lines, such as those derived from mouse myeloma (NS0), baby hamster kidney (BHK), human embryo kidney (HEK-293) and human retinal cells have gained regulatory approval for recombinant protein production.
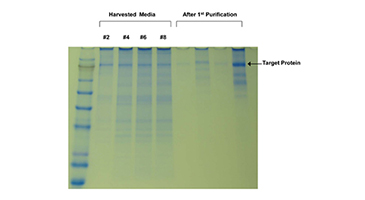
BHK cells had initial popularity as serum free mediums were developed early on to support their culture in suspension for scale-up. However, there were issues with productivity under serum free conditions in some cases. (1) FiberCell Laboratories cultured BHK cells expressing PEDF (pigment epithelium-derived factor, MW approximately 50 kD) using DMEM 10% FBS followed by a switch to DMEM 10% CDM-HD on day 17 with no adaptation. Data demonstrates stable glucose uptake rate and no loss of PEDF production when using CDM-HD. BHK cells can be cultured in hollow fiber bioreactors using protein free medium with no loss of production.
| Cartridge used | Cat #C2008 5 kD MWCO PS cartridge |
| Cells inoculated | 3 x 108 cells |
| Medium Consumed | 14 L |
| Total protein produced | 99.8 mg |
| Total volume of harvest | 164 mL |
| Average concentration | 0.6 mg/mL |
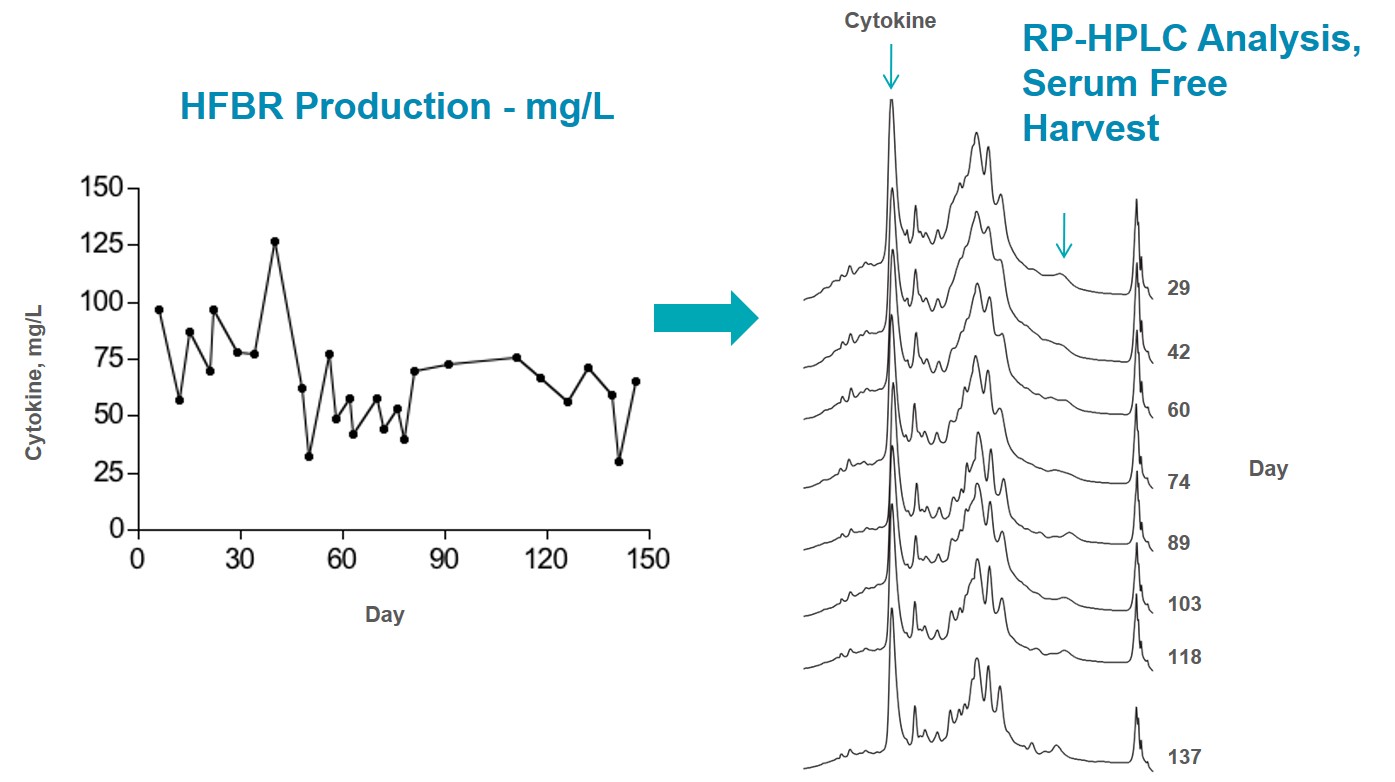
Culture was terminated at 22 days as the target of 100 mg of protein produced was reached.
The new generation of bio-therapeutics under development are increasing in size and complexity, with higher specific activities. As new therapeutic strategies are developed, bioengineering moves beyond copying that which is expressed naturally and begins to use novel and unique structures not found in nature, but created in the mind of the bioengineer. (3) This new class of proteins includes bi-specific T-cell engager (BITE) antibodies and tri-specific killer engagers (TRIKE) which utilize unique, newly created structures for their novel functionality. A HFBR system from FiberCell Systems allows any laboratory to take advantage of the superior folding, glycosylation and complete post-translational modifications that only expression in mammalian cells can provide. In vivo-like cell densities, constant provision of nutrients and removal of waste products, and total lack of shear result in complete and uniform posttranslation modifications over continuous production cultures. HFBRs are an effective method for producing milligram to gram quantities of recombinant proteins. The harvested product is concentrated and contaminating proteins, DNA, RNA, and proteases are reduced significantly. The use of CDM-HD renders the medium economical and chemically defined. Cultures can be maintained for long periods of time, meaning that scalability of the system is determined by length of culture, not new equipment. Protein-free medium and high concentration product simplifies downstream processing, and can result in increased yields by reducing the steps required for purification. A HFBR from FiberCell Systems is the ideal method for the production of 50 mg on up to gram quantities of recombinant proteins from mammalian cells, and is particularly useful for the production of difficult-to-express proteins.