High speed EV pellet from 275ml of harvested supernatant from the C2011 cartridge (so about 2 weeks of harvests). Yield is about 20mg. With FiberCell hollow fiber bioreactors you can see what you are working with.


Plastic trash generated by the culture of 1X109 cells

The C2011 cartridge can support large numbers of MSC and other cells, replacing 2-10 CellStacks.
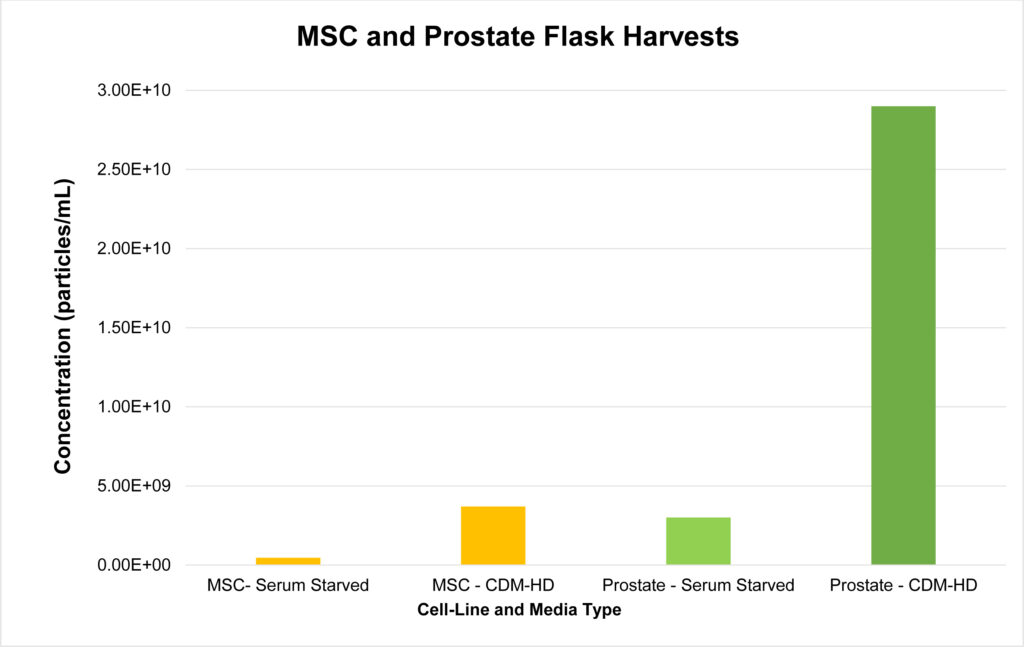
Harvests were taken from confluent T175 flasks with roughly 2.5×106 total cells in each flask. Serum starved flasks were grown up in 10% FBS+DMEM and serum starved for 48 hours prior to collection. CDM-HD media flasks were grown up in 10% FBS+DMEM and switched to 10% CDM-HD+DMEM for 48 hours prior to collection. Each flask contained 35 mL of media. Particle concentrations are as follows (particles/mL): MSC-Serum Starved (4.6×108), MSC-CDM-HD (3.7×109), Prostate-Serum Starved (3.0×109, Prostate-CDM-HD (2.9×1010).
Comparison of flask culture to a C2011 HFBR for the production of extracellular vesicles from mesenchymal stem cells
| Collection Volume (mL) | Total Exosome Protein (mg) | Total Exosome Particles (1010) | ||||
| HFBR | ||||||
| Cartridge #1 (7 weeks, collection every week) | 240 | 11.82 | 95.78 | |||
| Cartridge #2 (4 weeks , 6 collections) | 120 | 14.45 | 326.9 | |||
| Flasks | ||||||
| 130 T225 | 4000 | 0.9 | 1.6 | |||
Suggested Ordering For Extracellular Vesicles:
| Cartridges | ||||||||
| Catalog No. | Size | Surface Area | Fiber Type | Packing Density | ECS Vol | MWCO 50% | Max. Cell# | |
| C2025D | Small | 450 cm2 | High flux PS | 50% | 3.2 mL | 20 kD | 108 | more info |
| C2011 | Medium | 4000 cm2 | high flux PS | 50% | 15 mL | 20 kD | 109 | more info |
| C2018 | Large | 1.2 m2 | high flux PS | 50% | 70 mL | 20 kD | 5 x 1010 | more info |
| Reservoir Caps | ||||||||
| A1005 | 33 mm Reservoir Cap more info | |||||||
| A1006 | 38 mm Reservoir Cap more info | |||||||
| A1008 | 45 mm Reservoir Cap more info | |||||||


1. Comparison of flask culture to a C2011 HFBR for the production of extracellular vesicles from mesenchymal stem cells
5 X 108 human adipose derived adult MSC were cultured in a FiberCell Systems HFBR for 8 weeks using DMEM/10% FBS in the circulating medium only, with basal DMEM in the ECS. At the end of 8 weeks the cartridge was cut open and cells recovered for phenotypic analysis. This demonstrated no change in cell phenotype over the period of culture. See results here.
2. Production of IL-15 RC decorated EVs from HEK 293 cells using CDM-HD.
CD63 and Alix were greatly enriched from the bioreactor compared to flask culture. EV/protein ratio was 10-fold higher in harvests from the bioreactor suggesting a significant reduction in contaminating cell membrane fragments. Purified HEK 293 cells retained their IL-15 biological activity. See results here.