I have been using FiberCell Systems cartridges for the production of recombinant proteins in CHO cells for five years. I routinely produce a half-gram of protein in two months in a volume of less than five liters. This type of production would not be possible using conventional methods. FiberCell Systems hollow fiber bioreactors have made an invaluable contribution to my research.
– Dr. James Arthos, Bethesda, Md.
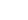
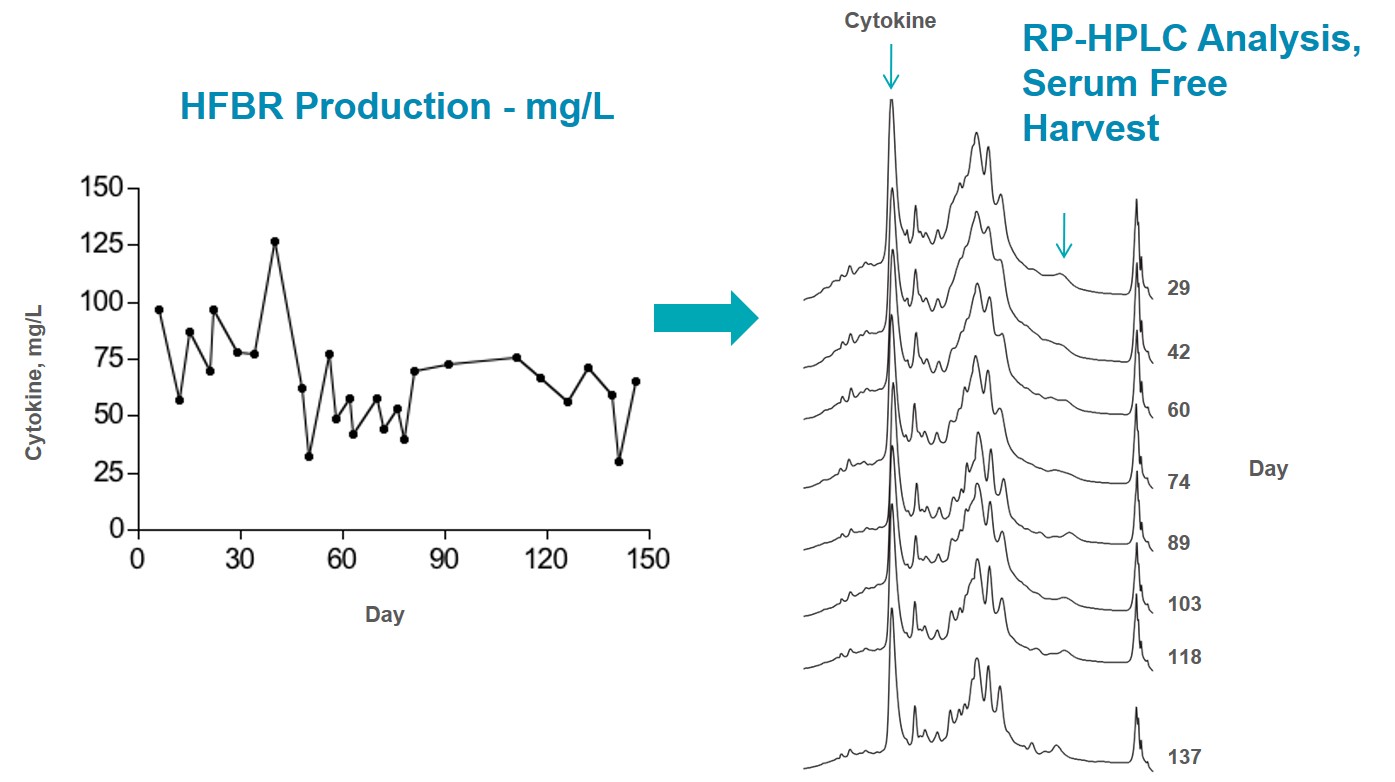
246 mg of purified recombinant IgG1 from a CHO (DG44) cell line was harvested from the C2011 20 kD MWCO cartridge. Medium was a serum free, protein free formulation similar to CDM-HD. Each harvest was 19 mL in volume; total harvest volume was 304 mL for an average concentration of over 800 micrograms per day per milliliter. The cartridge consumed 1 liter of medium every two days and the culture was maintained for a total of 35 days. Even though cell viability in the harvest was fairly low the expressed protein was very clean as demonstrated by the gel of the un-purified harvest shown below. Contamination with cellular proteins and DNA was quite low.
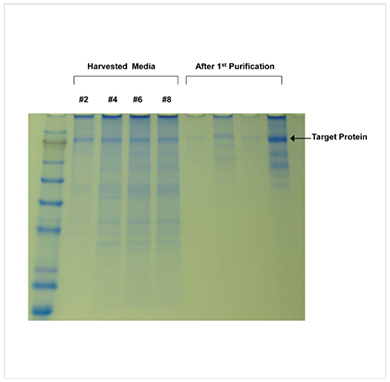
Recombinant immunoglobulin produced by 293t cells in our C2011 cartridge. This gel nicely demonstrates the reduction in contaminating serum proteins after switching to CDM-HD. In this case the researcher harvested every two days rather than every day, so the elimination of serum proteins takes a little longer. Note that the IgG is the dominant protein in the unpurified supernatant after the switch to CDM-HD is complete.
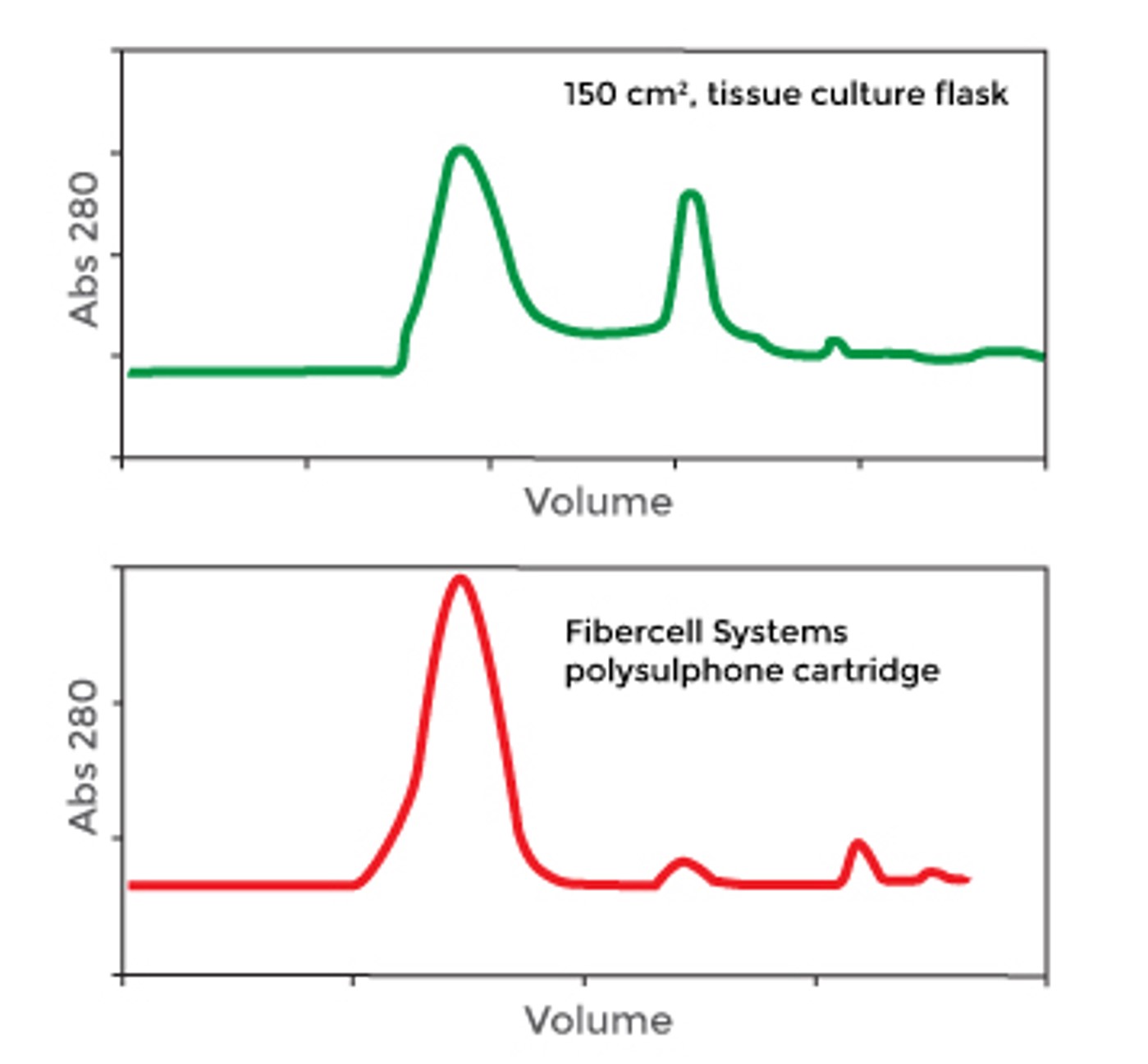
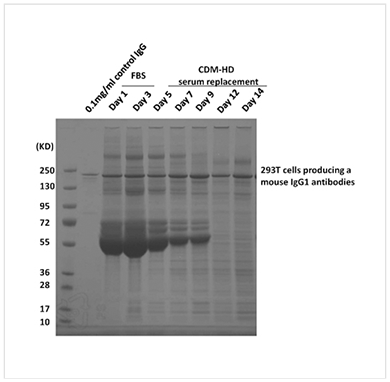
Hexeramized IgG expressed in CHO cells. 6 IgG subunits held together with 3 IgA tails. The Fv region has been replaced with CD4 receptor. Top trace is the protein expressed in 2-D flasks, bottom trace is the exact same cells, transferred into the hollow fiber bioreactor. In flask culture protein assembly is not complete, in the HFBR more than 90% of the protein is expressed as a properly folded hexamer.
- Both suspension and adherent cell types
- 100x+ higher concentration
- Easily adapt to SFM
- Can provide improved protein folding