When paired with our unique protein-free CDM-HD serum, the protein of interest becomes a significant component of the harvested supernatant and the small volumes facilitate purifications.
Interested to find out more about in-vivo-like environments? Click to read more.

Cartridge/Flowpath Specifications
| Catalog No. | Size | Surface Area |
Fiber Type | Packing Density |
ECS Vol | MWCO 50% |
Max. Cell# | Oxygenator |
| C2025D | Small | 450 cm2 | High flux PS | 50% | 3.2 mL | 20 kD | 108 | .65 m |
| C2008 | Medium | 4000 cm2 | Low flux PS | 50% | 20 mL | 5 kD | 109 | 4 m |
| C2011 | Medium | 4000 cm2 | High flux PS | 50% | 20 mL | 20 kD | 109 | 4 m |
| C5011 | Medium | 4000 cm2 | High flux PS | 50% | 20 mL | 20 kD | 2 x 109 | 6.1 m |
| C2003 | Large | 1.2 m2 | Low flux PS | 50% | 70 mL | 5 kD | 5 x 1010 | 6.1 m |
| C2018 | Large | 1.2 m2 | high flux PS | 50% | 70 mL | 20 kD | 5 x 1010 | 6.1 m |
| Bioreactor | ||||||||
| P3202 | The FiberCell Systems Duet more info | |||||||
| Cartridges | ||||||||
| Catalog No. | Size | Surface Area | Fiber Type | Packing Density | ECS Vol | MWCO 50% | Max. Cell# | |
| C2003 | Large | 1.2 m2 | low flux PS | 50% | 70 mL | 5 kD | 5 x 1010 | more info |
| C2008 | Medium | 4000 cm2 | low flux PS | 50% | 15 mL | 5 kD | 109 | more info |
| C2011 | Medium | 4000 cm2 | high flux PS | 50% | 15 mL | 20 kD | 109 | more info |
| C2018 | Large | 1.2 m2 | high flux PS | 50% | 70 mL | 20 kD | 5 x 1010 | more info |
| Reservoir Caps | ||||||||
| A1005 | 33 mm Reservoir Cap more info | |||||||
| A1006 | 38 mm Reservoir Cap more info | |||||||
| A1008 | 45 mm Reservoir Cap more info | |||||||
| Serum Replacement | ||||||||
| CDM-HD more info | ||||||||


1. Production of a Hexeramized Recombinant IgG from CHO Cells in the FiberCell Systems C2018 Cartridge
Production of a hexeramized IgG consisting of 6 IgG1 subunits held together by 3 IgA tails was expressed in a CHO cell line and produced in a 1.2 m2, 20 kD MWCO hollow fiber bioreactor. This is a classic example of a difficult-to-express protein; a very large, highly glycosylated, complex, engineered structure not seen in nature. See results here.
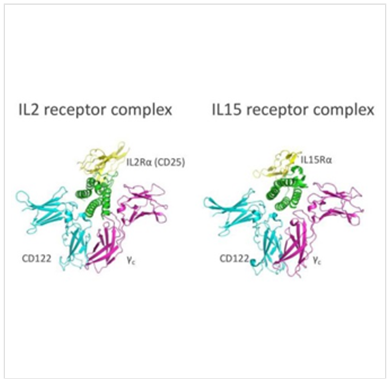
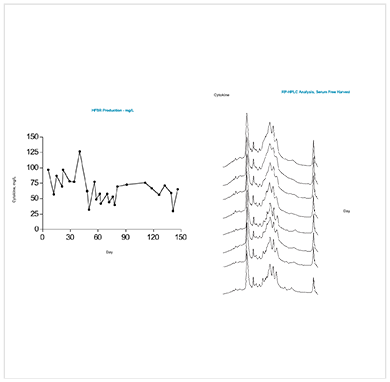
2. HEK 293 culture expressing heterodimeric IL-15 maintained in a HFBR for over 4 months of continuous production
Efficient production of this noncovalently linked but stable heterodimer in HEK293 cells is demonstrated in a 5 kD MWCO HFBR. Using the HFBR demonstrated consistent, continuous product of the IL over 150 days of culture with no change in glycosylation or conformation. See results here.
3. Recombinant IgG production in HEK 293 cells
Recombinant immunoglobulin produced by 293t cells in our C2011 cartridge demonstrated the reduction in contaminating serum proteins after switching to CDM-HD. See results here.
Interested to find out more? Read on for more case studies on recombinant proteins.